Pauels
Consulting
Scientific
Consulting, Medical Writing,
Drug Regulatory Affairs
Office
Steinfurt
Alleestr. 18
D-48565 Steinfurt
Germany
Phone: +49-(0)2552-9972-00
Fax: +49-(0)2552-9971-99
info@pauels-scientific.de
ENTER



©
Pauels Consulting 2008
in
identifying the optimal MAA strategy (as a Europe-based consultancy,
Pauels Consulting is specialized on national, decentralised, and
centralised European procedures),
Pauels
Consulting can assist you





in
organizing advice meetings with regulatory agencies,
in preparing briefing documents and presentations, as well as
preparation of meeting minutes,
in
coordinating MAA activities between project teams (e.g. quality,
nonclinical, clinical, pharmacovigilance, RA),
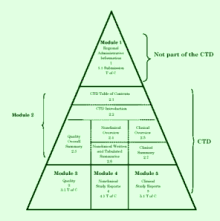
in IMPD and
CTD
compilation (paper form as well as eCTD), and transformation of older
documents into CTD format,
in
life-cycle management and uptating of documentations.
Regulatory
Consulting
Medical Writing
The success
of a Marketing Authorisation Application (MAA) depends on the chosen
application strategy, the communication with the regulators, the
keeping of time lines, and the quality of the submitted documentation.